Long Covalent Bond Theory
|
Long Covalent Bond Theory (LCBT) originated in January of 2020 upon deep analysis of α-quartz silica clusters (SiO2) via DFT computations. 37 months later, the first manuscript on LCBT was published in Frontiers of Chemistry. One driving force for inspecting silica was a poor understanding of its thermodynamic stability, despite being the most abundant substance in the Earth’s crust. A quantum-mechanical dissection of silica’s thermodynamics led to a strategy for its depolymerization, yielding alkylorthosilicates with the potential to be synthetic polymer building blocks (silica depolymerization paper, NSF native silicon grant). Another driving force was the realization that silica enjoyed resonance stabilization, but not via the resonance scheme that Pauling proposed in 1980 (Pauling 1980).
|
|
Miller, S. A. “The Location of the Chemical Bond. Application of Long Covalent Bond Theory to the Structure of Silica”
Front. Chem.,
2023,
11,
1123322.
https://doi.org/10.3389/fchem.2023.1123322
Graphical Abstract Caption. Molecular orbital analysis of model silica clusters reveals that over one-third of Earth’s valence electrons belong to long covalent oxygen-oxygen bonds, which conspire to stabilize silica via Möbius aromaticity.
|
|
What is the most abundant chemical bond on Earth?
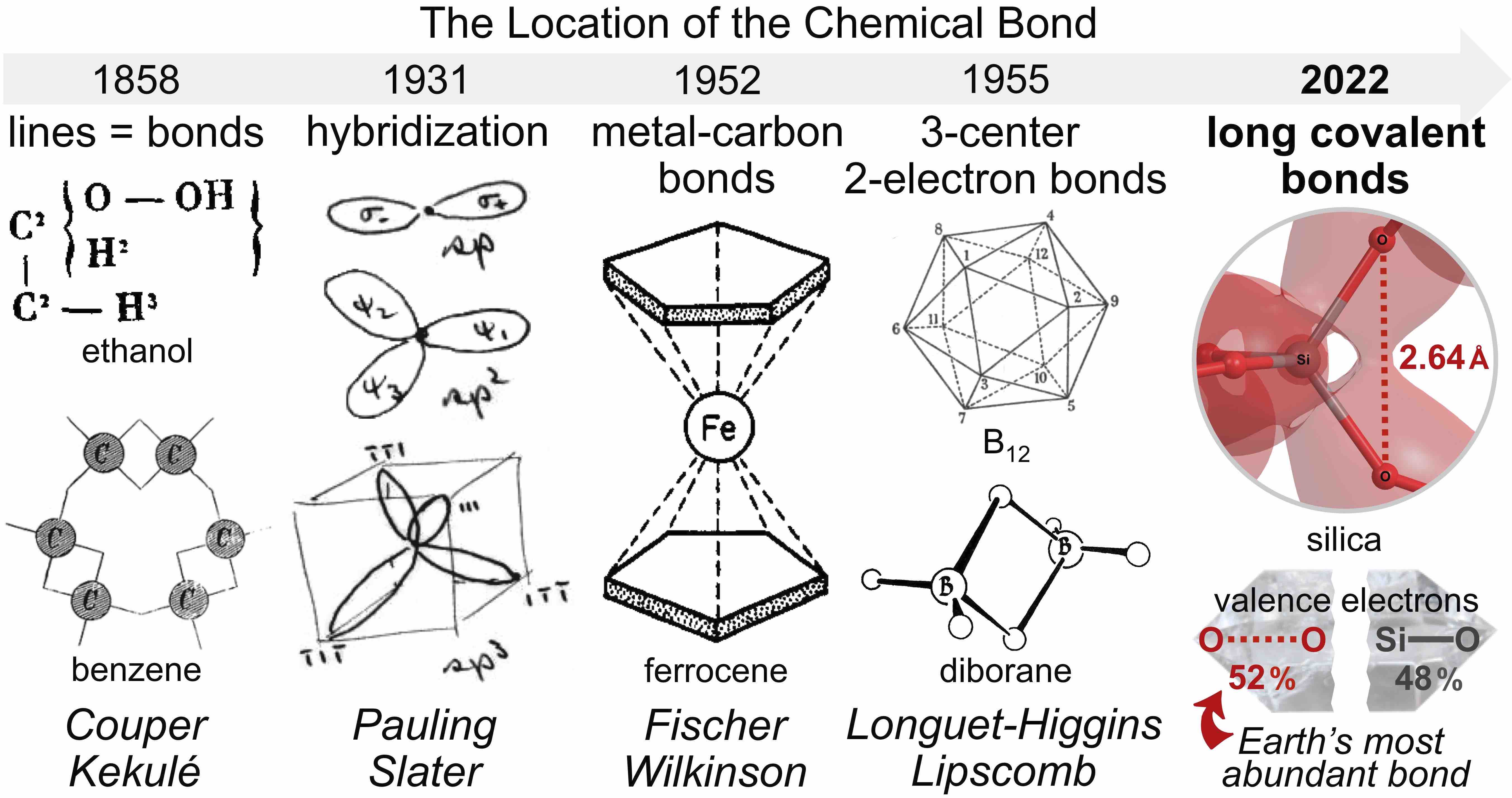
Since 1858 (Couper, Kekulé), the bonding in molecules and materials has been described by simplistic lines drawn between nearest atomic neighbors, somewhat like the connectors in a tennis racquet or volleyball net. The bonding in molecules and materials is vastly more complicated and resembles the intricate trestle of a bridge, with a variety of short and long connectors required to accurately describe the interactions between atoms. This manuscript introduces and defines Long Covalent Bond Theory (LCBT), a paradigm shift in our understanding of chemical bonding that directly challenges our antiquated and simplistic bonding model that limits covalent bond lengths to 2 Ångstroms. This theory is necessary to correct Pauling’s original resonance formulation for quartz silica, Earth’s most abundant material. The application of LCBT unravels longstanding mysteries of silica, including its stability, chirality (handedness), and valence bonding. Contrary to the present, simplistic lattice model that relies solely on silicon-oxygen bonding, silica enlists more valence electrons for oxygen-oxygen bonding (52%) than for silicon-oxygen bonding (48%). This newfound allocation means that silica’s oxygen-oxygen bond is the most abundant bond on Earth and employs over one-third of Earth’s 1049 crustal valence electrons. Moreover, application of LCBT is not limited to silica. This theory will provide great insight into the structure, bonding, and stability of countless molecules and crystals, including pharmaceuticals, ice, biopolymers, superconducting ceramics, and spaceflight materials, to name just a few.
|
|
Why is α-quartz chiral?
In 1811, optical activity was first discovered by François Arago in a sample of cristal de roche, quartz silica (Arago 1811). That momentous discovery has not been followed by an explanation for the chirality of quartz. A DFT computational molecular orbital analysis yields insight into the structure, stability, cooperative bonding, and chirality of α-quartz silica. Despite O-O distances of 2.61–2.64 Å, silica model complexes exhibit anomalously large O-O bond orders that increase with increasing cluster size—as the Si-O bond orders decrease. The large O-O bond orders are explained by an excess of O 2p - O 2p bonding interactions. Moreover, the isodesmic deconstruction of silica clusters reveals an O-O bond dissociation energy of 4.4 kcal/mol, summing to 26.4 kcal/mol of SiO2, which is the “extra stability” or resonance energy of silica. The Si6O6 twelve-membered rings of silica avoid achiral structures of high symmetry (e.g., D6h or C2v) because such rings would employ an equal number of bonding and anti-bonding oxygen-based molecular orbitals. Instead, the Si6O6 rings of α-quartz silica contort and twist to achieve chiral, C2-symmetry, allowing excess bonding molecular orbitals possessing Möbius aromaticity via long O 2p - O 2p bonds. After 212 years, the chirality of α-quartz silica can finally be explained—but only by understanding the long, non-canonical O-O bonds prescribed by Long Covalent Bond Theory (LCBT).
|
|
Thinking outside of the line
By drawing lines between atomic symbols, Couper and Kekulé created an extremely functional formalism but, after 160 years, it is time to think outside of the line.
A Nobel Laureate in Chemistry (to be identified later) was sent an early version of this manuscript (April 2022). I am grateful for the input and the three long emails, which include the following response: “You have a new way to look at what makes silicates so ubiquitous, so stable” and that “If this way of looking at silicates is of value, it will lead you to new chemistry. And you will be ahead of the people who are mired in the old way.”
|
Application of Long Covalent Bond Theory to the...
|
Coming soon...
Hint: What would Linus Pauling do with Spartan '24 DFT computational software and a couple of 32-core Mac Studio computers?
|
|
|
| |
|